
Mail
|
Search

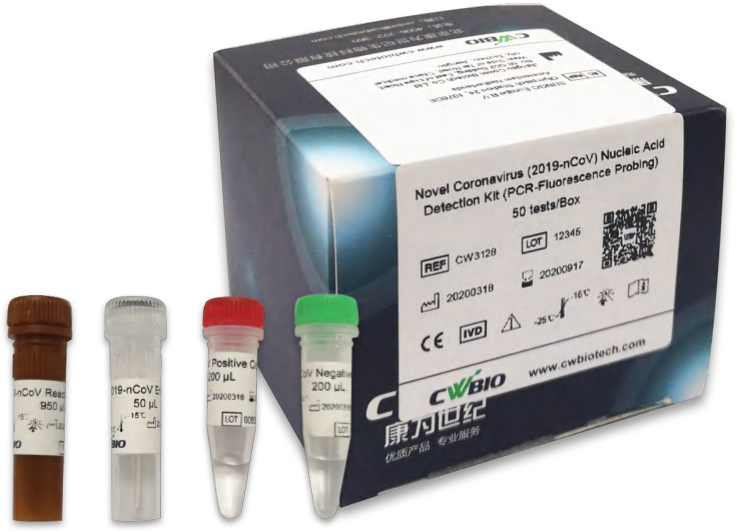
Novel Coronavirus (2019-nCoV) Nucleic Acid Detection Kit (PCR-Fluorescence Probing)
The Novel Coronavirus (2019-nCoV) Nucleic Acid Detection Kit is developed for qualitative detection of 2019-nCoV that is collected from human nasopharyngeal swabs, sputum and bronchoalveolar lavage fluid in vitro. It is only used as an in vitro diagnostic emergency response for 2019-nCoV infection happened in December 2019. It is suggested to follow the newest document instructions of “Diagnosis and treatment of novel coronavirus infection pneumonia” and “Strategic Preparation and Response Plan of novel coronavirus infection pneumonia” (current version). The novel coronaviruses belong to the β genus. 2019-nCoV is an acute respiratory infectious disease. People are generally susceptible.
Project Inquiry
- Advantages
- Specification
- Manual
